Randomly growing an embryo. It can work.
![]() Randomness. Shakespeare referred to it. The Bible talks about it.
People love to bicker about what it really is, or whether it truly
exists. And creationists, especially those of the ID subspecies,
consider it a fighting word. A random process, many would say, is a
process that doesn't involve God, or direction, or intention, or
whatever it is that the culture warriors of the Discovery Institute
are so foolishly fighting for. Ah, but it's not just the
propagandists of design-think who can mistakenly assume that an
ordered process is "directed." Consider this tale of a random
process being put to surprising use during vertebrate embryonic
development.
Randomness. Shakespeare referred to it. The Bible talks about it.
People love to bicker about what it really is, or whether it truly
exists. And creationists, especially those of the ID subspecies,
consider it a fighting word. A random process, many would say, is a
process that doesn't involve God, or direction, or intention, or
whatever it is that the culture warriors of the Discovery Institute
are so foolishly fighting for. Ah, but it's not just the
propagandists of design-think who can mistakenly assume that an
ordered process is "directed." Consider this tale of a random
process being put to surprising use during vertebrate embryonic
development.
Our story comes from Nature about a month ago, and I will present it in four acts.
Act I: The elongation of an embryo
We all know that animal embryos acquire their form through various morphings and twistings. One interesting example is axis elongation, which is just what it sounds like: the embryo stretches out until it clearly has a long axis, then continues to elongate to form something with a head and a tail and everything in between. But "stretch" is a poor term for what's really happening: the tail end of the embryo is growing while the structures closer to the head are beginning to develop into recognizable structures. Developmental biologists know that new cells are added near the tail end, and we know that various directed processes control many similar movements during early development. It was reasonable to assume that these mechanisms would account for embryo elongation, but the actual processes were unknown before the experiments of Bénazéraf and colleagues ("A random cell motility gradient downstream of FGF controls elongation of an amniote embryo," Nature 8 July 2010).
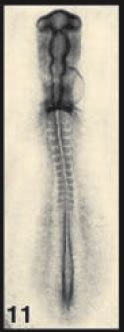
The authors employed an old warhorse of developmental biology, the chick embryo. At stage 11, the embryo looks nothing like the animal it will become; it has a head-like thing at one end (the top in the picture on the right), a weird hole at the bottom (Hensen's node), and some blocky structures called somites in between. Down at the bottom, on either side of the hole, is a tissue called the presomitic mesoderm (PSM). The anatomical details needn't concern us; what matters is that we understand that the embryo is elongating toward the bottom, that cells are being made near the top of that hole and that they are moving toward the tail, making it grow. Curious about how this works, Bénazéraf and colleagues started deleting pieces of the tail-end of the embryo, and they found that the PSM was critical for elongation. Good to know.
Act II: Cell movements in the elongating embryo
So, what's going on in the PSM that causes elongation? The authors used a nifty technique called electroporation to label the cells in that region so they could watch them as the embryo grew. Basically, they used an electric field to introduce DNA into the cells of interest the day before; the DNA caused the cells to express the wonderful and famous green fluorescent protein (GFP) so that individual cells could be monitored as the embryo continued to develop in culture outside of the egg. They found something interesting: near the tail of the embryo, the PSM cells were more motile than they were near the front of the PSM. But the cells near the front were more packed together. So try to picture it: in this region on either side of the center of the tail end of the embryo is an area (the PSM) of cells that are moving more frantically near the tail and that are more packed together toward the head. It would seem as though the cells are busily moving toward the tail, and that they get less crowded and more mobile as they get there. And when the authors looked at movement of individual cells, sure enough, there was a directional bias in the movement, meaning simply that cells in the PSM tended to move toward the tail. It looks like a simple case of directed migration of cells toward a target. Interesting, maybe, but not such big news. But then, a noise from the next room. Exeunt.
Act III: Random cell movements in the elongating embryo
So cells seem to move toward the tail. This could mean they're being directed toward the tail by some kind of homing mechanism, and this would be a reasonable expectation. But because the embryo is elongating, it could be that the directed movement of individual cells is an illusion: the cells are moving toward the tail because the space they inhabit is moving toward the tail. The authors addressed this by cancelling out the effect of elongation of the cells' environment, and focusing solely on the movement of cells within that environment. The environment in this case is the extracellular matrix, or ECM, as indicated by one of its components, fibronectin. I'm sorry about the jargon, but I included it so I could quote the authors in full as they describe the results of the experiment:
Surprisingly, the movements of cells relative to the ECM did not show any local directional bias. The mean square displacement of these cells compared to the fibronectin movement scales with time, indicating that cells exhibit a 'random walk'-like diffusive behaviour, with the diffusion of cells relative to the fibronectin following a posterior-to-anterior [back-to-front] gradient.
In other words, the cells are moving randomly, behaving like molecules diffusing in a liquid. The authors verified this by looking at cell protrusions, the telltale signs of a cell's migrational direction. The protrusions all pointed in random directions. Amazingly, this seemingly ordered march of cells toward the back, resulting in the growth of the tail end of the embryo, is the product of random cell movement. And yet it yields an ordered result. How?
Act IV: A gradient of random cell movement controlled by a conserved developmental signaling system
Recall that cell movement in the PSM is not uniform: cells near the tail move (randomly) more. The authors knew that an ancient and well-known signaling system functions in a similarly graded fashion in that tissue. Known as the FGF/MAPK pathway, it's fairly simple to manipulate experimentally. Bénazéraf and colleagues found that whether they turned the signaling up or down, the result was the same: elongation was stunted. This might seem strange, but it makes perfect sense: it's the graded nature of the signaling that matters, so turning it all the way up or all the way down erases the gradient and leads to the same result. What matters, for elongation, is that random cell movement is greater in the back than in the front. This leads to elongation, because the tail end contains cells that move more and have more freedom of motion due to their being less tightly packed.
The upshot is that an ancient conserved signaling system causes a simple gradient of random movement which, in the presence of physical constraints, leads necessarily to elongation of the embryo in one direction. It looks for all the world like homing or some other directed migration, but it's not. And, intriguingly, the authors conclude by suggesting that the mechanism might be quite common in the biosphere:
Axis formation by outgrowth is a common morphogenetic strategy that is widely evident in animals and plants. Thus, the mechanism described here might apply to other well-characterized, polarized axes, such as the limb buds, in which a similar FGF/MAPK gradient is established along the proximo-distal axis.
Randomness. Learn to love it. The End.
-----
Image credit: "Normal stages of chick embryonic development," poster on Developmental Dynamics site
Bénazéraf, B., Francois, P., Baker, R., Denans, N., Little, C., & Pourquié, O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo Nature, 466 (7303), 248-252 DOI: 10.1038/nature09151