Down with phyla!
 Are phyla “real”? Is there really a well-defined “number of animal phyla” extant and in the fossil record? Does the term “bodyplan” or “bauplan” have any consistent definition? Many paleontologists, notably Stephen Jay Gould (1989, Wonderful Life), have written books that take these concepts for granted, and, observing charts with many animal phyla appearing in the Cambrian, and few appearing afterwards, have reached the conclusion that there was something extra-special and unique about the Cambrian “explosion”. Creationists, both the traditional and “intelligent design” variety, have been only to happy to put their own spin on this situation, and argue that God, for reasons that remain obscure, engaged in a particularly active period of special creation for a few dozen million years back in the Cambrian. Recent examples include Stephen Meyer’s hopeless paper “The origin of biological information and the higher taxonomic categories”, the three or so previously-published versions of that paper, and Paul Nelson’s work in general (see a recent powerpoint presentation).
Are phyla “real”? Is there really a well-defined “number of animal phyla” extant and in the fossil record? Does the term “bodyplan” or “bauplan” have any consistent definition? Many paleontologists, notably Stephen Jay Gould (1989, Wonderful Life), have written books that take these concepts for granted, and, observing charts with many animal phyla appearing in the Cambrian, and few appearing afterwards, have reached the conclusion that there was something extra-special and unique about the Cambrian “explosion”. Creationists, both the traditional and “intelligent design” variety, have been only to happy to put their own spin on this situation, and argue that God, for reasons that remain obscure, engaged in a particularly active period of special creation for a few dozen million years back in the Cambrian. Recent examples include Stephen Meyer’s hopeless paper “The origin of biological information and the higher taxonomic categories”, the three or so previously-published versions of that paper, and Paul Nelson’s work in general (see a recent powerpoint presentation).
Last week I came across the following paper:
David Fitch and Walter Sudhaus, “One small step for worms, one giant leap for ‘Bauplan?’” Evolution & Development 4:4, 243-246.
The paper is a frontal attack on the concepts of “phyla” and “bodyplan,” especially as applied to Cambrian fossils.
Nematode mouths
The paper begins with nematodes. Nematodes are a ubiquitous group of (usually) tiny worms. They live in the soil, in the ocean, and in and on many other metazoan animals, including you. There are so many nematodes around that it has been said that if all multicellular life except nematodes were to suddenly vanish, you would still be able to see ghostly images of plants, animals, and humans – made up entirely of nematodes. Nematodes are bilateral metazoans, recently placed in the ecdysozoa, a group of phyla that molt their cuticles. Arthropods and sister phyla such as tardigrades and onychophorans are also ecdysozoans.
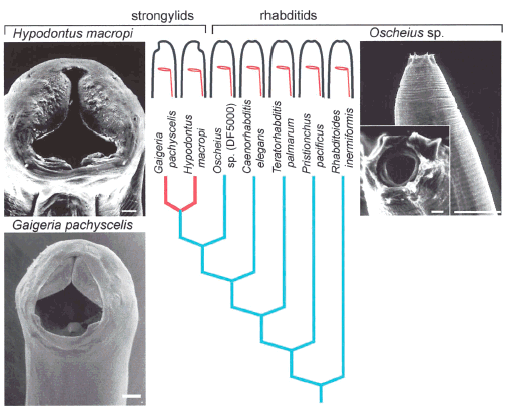
Fitch and Sudhaus (2002) begin by noting that in certain lineages of nematodes, the mouth has shifted from a terminal position (“terminal” means that the mouth is at the front tip of the worm, to a “neural” position (twisted to be on the same side of the body as the neural cord) or to an “abneural” position (twisted to be on the opposite side of the neural chord). See their Figure 1a:
Note: This figure shows three nematode species, Hypodontus macropi (mouth is neural), Gaigeria pachyscelis (mouth is abneural), and Oscheius sp. (mouth is terminal). Red indicates the appearance of the character change in the phylogeny.
This shift in mouth position is a quite minor change, and has happened independently in various taxa.
Phyla-level differences
Now, one of the “key differences” separating chordates (deuterostomes) from protostomes is that the chordate mouth is abneural, while the protostome mouth is neural. Chordates, echinoderms, and some other wormy phyla are deuterostomes, while the other major group of “advanced” bilaterian animals are the protostomes, which include the ecdysozoans we just met as well as the lophotrochozoans (mollusks, annelids, and others). The differences between protostomes and deuterostomes are supposed to be even “bigger” than the differences between phyla within these groups (after all, each of these groups includes many phyla). The character differences between the phyla are considered to be a fundamental parts of the “bodyplans” of the various phyla.
Fitch and Sudhaus note, however, that this key character change has occurred many times, in nematodes and elsewhere, and in these contexts it is considered a minor change – perhaps warranting a new family or genus, but certainly not a new “bodyplan” or phylum. But what is the meaning of “bodyplan” and phylum, if “phylum-level” character changes are going on continually during the history of life, and these changes are considered minor except in the context of the phyla?
Fitch and Sudhaus conclude that – rather like a “language” is a dialect with an army, and the difference between a “religion” and a cult is about 100 years – a “phylum-level difference” is a small set of changes that occurred when animals were first diversifying. These changes were not particularly “fundamental” or radical at the time, they were just early. All of the latter changes that accumulated in each lineage were built upon these early changes, producing the appearance – to modern eyes – of these changes being “fundamental differences”. Among modern organisms, the phyla are fairly distinct now due to accumulated changes and extinction of basal stem groups [1]. But when the phyla were first diverging, the differences were not so large, and many of the stem groups were still around. This is the reason why many of the Cambrian fossils are difficult to categorize. If we attempt to shoehorn them into modern taxa, many of them don’t fit, so we have to erect new phyla for them, even though the morphological difference between (say) a lobopod and a basal fossil arthropod or basal fossil onychophoran is not large.
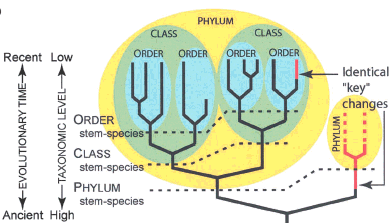
Fitch and Sudhaus show this inconsistency in their Figure 1b:
Note: This figure shows that in Linnaean taxonomy, not all character changes in organisms are treated equally, even if they are identical changes. (Red = new character on the phylogeny)
Linnaean taxonomy works passably well on modern organisms – each modern organism can be put into a natural hierarchy of monophyletic groups [2]. But when applied to fossils, internal contradictions and absurdities begin to appear. For example, let’s say that we define the class “Aves” as the common ancestor of Archaeopteryx and modern birds, and all of that common ancestor’s descendents. So far, so good. However, when we discover a flock of feathered theropod dinosaur fossils, some more closely related to Archaeopteryx than others, we are struck with a quandary. In order to be self-consistent, the sister group of (Archaeopteryx+modern birds) should itself be ranked as a class. The sister group of these two classes should get a rank higher than class. However, these feathered theropods are clearly just one small group of dinosaurs, and if they were all transported to modern times, they are so similar we would put them all in the same small group. The only ways out of this bind are to (1) give up on the requirement that groups be monophyletic (this is what paleontologists used to do, e.g. with the “mammal-like reptiles”, (2) give up on self-consistency of ranking (leading to problems like having a class within a family, e.g. with the bird-dinosaur example above), or (3) give up on the Linnaean system altogether for fossils, and simply assign a specimen to a species and then determine whether or not it belongs to a crown group (nested within group defined by the common ancestor of a modern monophyletic group) or a stem group (on a branch basal to a crown group).
Option 3, the cladistic option, has taken over much of paleontology, e.g. studies of the origin of birds or the origin of mammals. Fitch and Sudhaus (2002), and several other papers quoted below in an appendix to this post, represent this view taking over Cambrian paleontology.
Implications for ID/creationist argumentation
The implications of this conceptual shift within mainstream Cambrian paleontology for ID/creationist argumentation with respect to the Cambrian “explosion” of “phyla” are rather dire. The entire basis for the ID position is revealed to basically be a mistake – a mistake made by eminent evolutionary biologists, to be sure, but a mistake nonetheless. The “extinct phyla” that Gould and others cited in support of the idea that the Cambrian phyla appeared in a “phylogenetic lawn” are rapidly being placed as stem groups of modern phyla, showing us how the characters of modern phyla were acquired step-by-step.
There is, of course, zero chance that IDists will just give up on the beloved Cambrian Explosion, but alert creationism watchers might see them move the goalposts. I suspect we are already seeing some of this when we see IDists waffle on whether or not the Cambrian explosion was really that abrupt, and whether or not any transitional fossils for Cambrian phyla really exist. It appears that attempts at “in-principle” claims are being substituted – for example, instead of “the Cambrian explosion happened too fast for evolution, too many new body plans with no transitionals”, we are seeing things like “evolution can’t produce new information or new developmental programs, no matter how gradual the Cambrian Explosion was.” Two examples are quoted below:
Those who think the fossil data provide a more reliable picture of the origin of the Metazoan tend to think these animals arose relatively quickly–that the Cambrian explosion had a “short fuse.” (Conway Morris 2003b:505-506, Valentine & Jablonski 2003). Some (Wray et al. 1996), but not all (Ayala et al. 1998), who think that molecular phylogenies establish reliable divergence times from pre-Cambrian ancestors think that the Cambrian animals evolved over a very long period of time–that the Cambrian explosion had a “long fuse.” This review will not address these questions of historical pattern. Instead, it will analyze whether the neo-Darwinian process of mutation and selection, or other processes of evolutionary change, can generate the form and information necessary to produce the animals that arise in the Cambrian. This analysis will, for the most part, therefore, not depend upon assumptions of either a long or short fuse for the Cambrian explosion, or upon a monophyletic or polyphyletic view of the early history of life.
Stephen C. Meyer (2004), “The Origin of Biological Information and the Higher Taxonomic Categories”
But the puzzle of the Cambrian Explosion is not really a paleontological (i.e., fossil) problem.
The fossils just make the puzzle more dramatic.
The real problem arises from the way that animals are constructed by the process of development. (emphasis original)
Paul Nelson (2005). “Why is the Problem of Macroevolution Still Unsolved?” PowerPoint presentation, University of Minnesota-Morris, 6 April 2005.
In the Appendix below I will quote the relevant bits of some recent articles that make the points I tried to outline in my post above, but in much more authoritative and rigorous fashion.
Notes
-
However, it is worth reading Valentine’s (2004) book The Origin of Phyla and noting every time Valentine says things like “group X is currently placed in phylum A, but it used to be its own phylum B, and before that it was a subphylum in phylum C, but phylum C had to be discarded as a polyphyletic ragbag.”
-
There are some issues with losing phylogenetic resolution (not all of the splits in a phylogenetic tree can be given a rank, even if we start assigning suborders and superclasses) and with the false assumption that taxonomic ranks are going to be comparable (the tree genus Nothofagus is over 60 million years old, the genus Homo is only a few million years old).
-
Well, this note isn’t attached to anything, but I should add that there are some reasons that the origin of the Cambrian phyla is different than the origin of birds or mammals. First, it was the “mother of all adaptive radiations”, occupying niches that were not just open but completely unoccupied. Second, there was a major change in the environment for fossil preservation, notably (a) the origin of hard body parts and (b) the origin of burrowing, algae-scraping, and other forms of muck-sucking that mixes up the sediment. The pre-Cambrian world appears to have been one of undisturbed algal mats, until advanced metazoans came along to eat all of that up. So calling the Cambrian “explosion” a “myth” – referring to the “phylogenetic lawn” idea – does not exclude the fact that some some very interesting and important things happened at the beginning of the Cambrian.
Appendix – “Down with phyla” excerpts from recent scientific articles
David Fitch and Walter Sudhaus, “One small step for worms, one giant leap for ‘Bauplan?’” Evolution & Development 4:4, 243-246.
[p. 243]
A popular hypothesis about animal diversification is that unique changes occurred in the Precambrian or Cambrian (ca. 700-500 millions of years [Myr] ago) to produce the distinctive features of all animal “Baupläne” (“body plans”) and that such changes have not occurred since (Gould 1989:47). In contrast, we suggest that changes similar to the key innovations initiating the appearance of these distinctive features occur repeatedly during evolution. A major example is the “inversion” of the dorsoventral axis in the evolution of chordates (Arendt and Nübler-Jung 1994), initiated by a switch in mouth position from the neural to the abneural side. Here we note that similar changes in mouth position evolved less than 50 Myr ago at least twice in a group of nematodes related to Caenorhabditis elegans. Because this means that such changes were not unique to the Cambrian, they can be studied by experimental approaches in closely related extant organisms. A direct consequence of this focus on studying elemental key changes is that “Bauplan” becomes a less useful concept for understanding how animal diversity evolved.
As a practical approach to understand the origin of differences between currently disparate forms, we can analyze these differences in terms of the suites of apomorphic (derived) evolutionary changes that made one form different from an other. Such disparity resulted from many accumulated alterations, novelties, and reductions and the extinction of animals from side lineages with intermediate forms (Sudhaus and Rehfeld 1992:185-188). Retrospectively, some of these changes (which we call “key” changes) might be considered more important than others in initiating a major difference. Even slight changes could provide the important first step (retrospectively recognized as key) in an evolutionary series of events resulting in a major difference between taxa. This approach of identifying key changes relieves us from dealing with Bauplan (body plan), which is typological and has un certain ontology. (Bauplan has been defined as a “phylotypic” organization or archetypal pattern shared by species in a supraspecific taxon and that is distinguishable from other such patterns; e.g., it is unclear how many differences of what grade distinguish Baupläne [Gerhart and Kirschner 1997:296; Raff 1996:33; Sudhaus and Rehfeld 1992:185].) In fact, a break with such typology was the foundation for Darwin’s revolutionary conceptual framework (Mayr 1979). Epistemologically, identifying key changes is more likely to give us a practical understanding of the origins of morphological disparity than trying to fit variation into typological concepts like Bauplan.
[…]
[p. 244]
Why is it not recognized more widely that many of the kinds of changes ultimately leading to disparate forms were not unique to the Precambrian/Cambrian? One reason may be that the human mind is so impressed with large differences that it cannot easily conceive origins of such differences in small steps (see Darwin 1859:29). Perhaps focusing on typological Baupläne exacerbates this difficulty? But a more important reason is the common misconception (also sustained by typological terms like “phylum-level body plan,” “phylotypic stage,” and “phylotypic process”) that the taxonomic level of Phylum is primarily determined by Bauplan (or developmental stage or spatial pattern of develop mental regulatory mechanisms). First, it is tautological to use Bauplan to define a particular taxonomic level if a Bauplan is itself defined as the set of features characteristic of a particular taxon. Second, it has been considered “paradoxical” that “all phyla are old” despite “repeated opportunities for the appearance of new phyla” (Raff 1996:174). This paradox is resolved by noting that the different hierarchical levels of the taxonomic system (Phylum, Class, Order, etc.) are applied arbitrarily. These taxonomic levels reflect relative divergence points in time, as Darwin (1859:420) famously recognized, not particular differences in Bauplan. That is, the groups-within-groups hierarchy of taxonomy simply derives from common ancestry at more and more ancient times (Fig. 1B). Phylum divisions represent divergences that occurred earlier than Class or Order divisions within the Phylum, regardless of the grade of difference in Bauplan (Darwin 1859). Even if an identical key innovation as that characterizing a “phylum-level body plan” arose recently from within an Order, a new Phylum could not be erected for it without upsetting the entire taxonomic hierarchy, no matter how distinct the new Bauplan (Fig. 1B). Thus, “all phyla are old” simply because of the hierarchical restrictions of taxonomy, not because fundamental key changes to body plans have not arisen more recently. A paucity of Phyla more recently emerged than the Cambrian is therefore not evidence for lack of recent innovative changes in Bauplan.
Budd, G. E. and S. E. Jensen. 2000. “A critical reappraisal of the fossil record of the bilaterian phyla. Biological Reviews of the Cambridge Philosophical Society 75:253-295.
[p. 253]
ABSTRACT
It has long been assumed that the extant bilaterian phyla generally have their origin in the Cambrian explosion, when they appear in an essentially modern form. Both these assumptions are questionable. A strict application of stem- and crown-group concepts to phyla shows that although the branching points of many clades may have occurred in the Early Cambrian or before, the appearance of the modern body plans was in most cases later : very few bilaterian phyla sensu stricto have demonstrable representatives in the earliest Cambrian. Given that the early branching points of major clades is an inevitable result of the geometry of clade diversification, the alleged phenomenon of phyla appearing early and remaining morphologically static is seen not to require particular explanation. Confusion in the definition of a phylum has thus led to attempts to explain (especially from a developmental perspective) a feature that is partly inevitable, partly illusory.
[…]
[p. 255]
II. WHAT, IF ANYTHING, IS A PHYLUM?
Although the debate about the origins of phyla has been vigorously conducted, there has been surprisingly little debate about the very terms of enquiry: how is a phylum defined, and how would variations in its composition change the nature of the debate? (See Valentine & Hamilton, 1997 for a useful exception.) There is, in fact, a difference between those who see a phylum as ‘a group of species sharing a common organization of the body’ (Adoutte et al., 1999, p. 104) and those who see a phylum in phylogenetic terms, although the problem is concealed by the common assumption that members of a phylum are in some way united by a body plan. Nevertheless, in the extant fauna, phyla appear to be used as the largest groupings of taxa that can readily be seen to be more closely related to each other than to any other groups: a primarily taxonomic or phylogenetic usage rather than a morphological, ‘body plan’ based one [for some phyla are recognized to include highly aberrant members, such as the pentastomids (Abele, Kim & Felgenhauer, 1989), or Xenoturbella (Israelsson, 1997; Noren & Jondelius, 1997)], although, critically, such assessments have generally been based on morphology. Claims that the phyla are characterized by particular types of ‘body plan’ features which putative super-phyletic groupings do not possess (e.g. see Table 2-2 in Arthur, 1997) thus seem to be based on an artifact of how we classify groups of animals: if such ‘super-phyletic’ features were readily identifiable, the larger grouping would itself probably be called a phylum, as it would be recognized to be phylogenetically unified. As the level at which this ignorance of relationships becomes important is likely to vary between groups, the cladist’s standard criticism that phyla (and other such ranks) should be positively discouraged on the grounds that they engender spurious comparisons between members of the same ‘rank’ (see e.g. Smith, 1994, and references therein) seems to be valid.
Whilst phyla are often satisfactory and coherent groupings, an important corollary of this usage is that phyla are defined in such a way that virtually guarantees we are ignorant about their interrelationships. Indeed, morphologically distinct groups of taxa that nevertheless do show clear affinities to one or other of the major phyla (such as the onychophorans, tardigrades, acanthocephalans, pogonophorans or echiurans) present a problem for the phylum concept (Budd, 1998a). Such groups are sometimes referred to as arthropods, rotifers or annelids, and sometimes as phyla in their own right. Such difficulties demonstrate the tensions that arise from trying to think about phyla in both phylogenetic terms and in terms of a group of taxa which share a particular ‘body plan’. Given that the phyla have an evolutionary origin, their characters ± and thus their body plans ± must in broad terms have been assembled in a particular order (Valentine & Hamilton, 1997; Budd, 1996, 1998a). There is thus a logical decoupling between the body plan that the extant members of a phylum share, and their phylogenetic affinities to each other, even when they are tightly correlated with each other in the extant fauna. Early in the history of a clade, when the body-plan features of a group had in the main yet to emerge, members of sister-group lineages of different clades must have been very similar to each other (see e.g. Erwin, Valentine & Jablonski, 1997). This distinction is crucial, because confusion between the phylum considered as a phylogenetic grouping and as a group of taxa that share a body plan has led to considerable misinterpretation of the evolutionary origins of phyla.
[…]
[p. 287]
Indeed, recent emphasis on the Ordovician radiation, which in some accounts is as significant as the Cambrian one (Droser, Fortey & Li, 1996) is entirely in accord with this view. Phyla may be a useful way of viewing the diversity of
[p. 288]
extant taxa, but become a typological hindrance in understanding its origin. Virtually all zoology text books perpetuate this problem by referring to the ‘sudden origin of phyla at the base of the Cambrian’, a misinterpretation of the fossil record based on this sort of typology.
Graham Budd (2001). “Climbing life’s tree.” Nature 412, 487.
[p. 487]
Fossils have always been a bother. Initially, natural philosophers were more impressed by their stony composition and where they were found than by what they looked like. Accordingly, they were compared to gemstones as often as to living organisms - perhaps not the best start for palaeobiology. Even when fossils were recognized as the remains of past life, no one knew how to classify them. Dinosaurs, ammonites and trilobites seemed to be quite like other reptiles, cephalopods and arthropods. But which ones were they like in particular? Conscientious palaeontologists strained sinews trying to force these groups to behave. Surely trilobites were a type of crustacean? Or did those antennae make them insects?
As these efforts at classification often failed, palaeontologists changed tack, creating countless high-level categories for fossils. At best, problematic groups were tagged as, for instance, ‘annelid-like’, given their own class or phylum, and cheerfully connected to the tree of life with dotted lines and question marks. This gave rise to the view that early evolution was different from ‘standard’ microevolution, with living groups of organisms suddenly appearing amid fireworks of excess ‘body plans’. The most popular victim of this muddle has undoubtedly been the origin of animals in the ‘Cambrian explosion’. Yet this amazing pattern - the inspiration for entire books devoted to analyses of its supporting mechanisms - is entirely the consequence of bad systematics.
Jaume Baguñà And Jordi Garcia-Fernàndez (2003). “Evo-Devo: the Long and Winding Road.” Int. J. Dev. Biol. 47: 705-713. PubMed
[p. 708]
Another stumbling block to get a balanced assessment of macroevolution is the excessive, almost mystical, adherence to typological concepts such as Baüplan and phylum which are preformationist and pre-evolutionary. Such concepts muddle and distort the perception of big radiations (the paradigm is the so-called Cambrian Explosion, though it could be extended to the radiation of land plants, mammals, etc,…) leading us to see them as something amazing, exceptional and unique, which they were not, and needing exceptional mechanisms, which likely were not required. Budd (2001b) and Fitch and Sudhaus (2002) have cogently argued (see also Conway-Morris, 2003) that such perceptions are the result of bad systematics (‘stem groups’ or fossils are usually left out) and of not considering that with elapsed time both the disparity among clades and the opportunity for extinctions of intermediate forms increase. Skipping the fossil record removes the ‘stem groups’ (those between the most recent common ancestor of two living groups and that of only one of them), which must comprise, by definition, only fossil organisms. This leaves for comparison only ‘crown groups’ (the most recent common ancestor of a clade plus all of its descendants) which are of little help, especially when comparing high clades (e.g. phyla). This is because lineages diverged from each other in a step-by-step manner which is only documented in the fossil record. In addition, ignoring that elapsed time increases the opportunity for intermediate forms to be extinct, reinforces the mirage that extant
[p. 709]
‘crown groups’ (usually phyla) appeared at once in their present modern form.
Walter Sudhaus (2004). “Radiation within the framework of evolutionary ecology.” Organisms, Diversity & Evolution 4, 127-134.
[p. 128]
For paleontologists radiation is a ‘macroevolutionary’ phenomenon. When looking at fossils, a new bauplan is found to be built up within a relatively short geological period of some tens of millions of years (e.g. high-rank groups of birds and presumably eutherian mammals in the Upper Cretaceous period before the K/T event, and the radiation of these groups after this period of mass extinction). Such data have led to the image of a sudden and “explosive” radiation, the “more or less simultaneous divergence of numerous lines” from an ancestor (Simpson 1953, p. 223), like exploding fireworks that suddenly and simultaneously burst in all directions. This image has become so deeply ingrained in the thinking of evolutionists that nearly no one questions myths like the “Cambrian explosion” (Fitch and Sudhaus 2002).
This last paper is less excited, but does indicate that the nematode mouth change is not unique:
Gonzalo Giribet (2003). “Molecules, development and fossils in the study of metazoan evolution; Articulata versus Ecdysozoa revisited.” Zoology 106: 303-326.
[p. 312]
Most arthropods have mouths that are situated ventrally or subventrally and directed posteriorly, possibly through caudal rotation of the mouth cone (Dewel et al., 1999) independent of that of onychophorans (Eriksson et al., 2003). Primitive arthropods such as Kerygmachela and many lobopodians had terminal mouths, with either unassisted or frontal appendage-assisted feeding. One idea is that arthropods later switched to predatory thoracophagy in some anomalocaridids and most euarthropods (Dewel et al., 1999; Budd, 2002). However, members of the extant Pycnogonida have their mouths located at the terminal end of a proboscis (King, 1973), both as juveniles and as adults. This is indeed interesting because by some authors pycnogonids are considered the sister group of all the remaining extant arthropods (Zrzavy et al., 1998a; Giribet et al., 2001). Considering the information from extinct arthropods and lobopodians, as well as tardigrades and pycnogonids, the putative sister group of the remaining arthropods, it seems that the terminal mouth opening could constitute a plesiomorphic state of panarthropods and an apomorphy of Ecdysozoa. This feature would have been lost in the non-pycnogonid arthropods as well as in modern onychophorans and in certain lineages of nematodes (Fitch and Sudhaus, 2002).